Jun 9, 2020 in Tutoring
Radiation Physics
This article is directed at students looking to study Physics at Secondary School Level. The article centres around Rutherf
It's your turn now! Let's support each other by clicking "Helpful".
+1
DISCUSS #Relationship
DISCUSS #Parenting
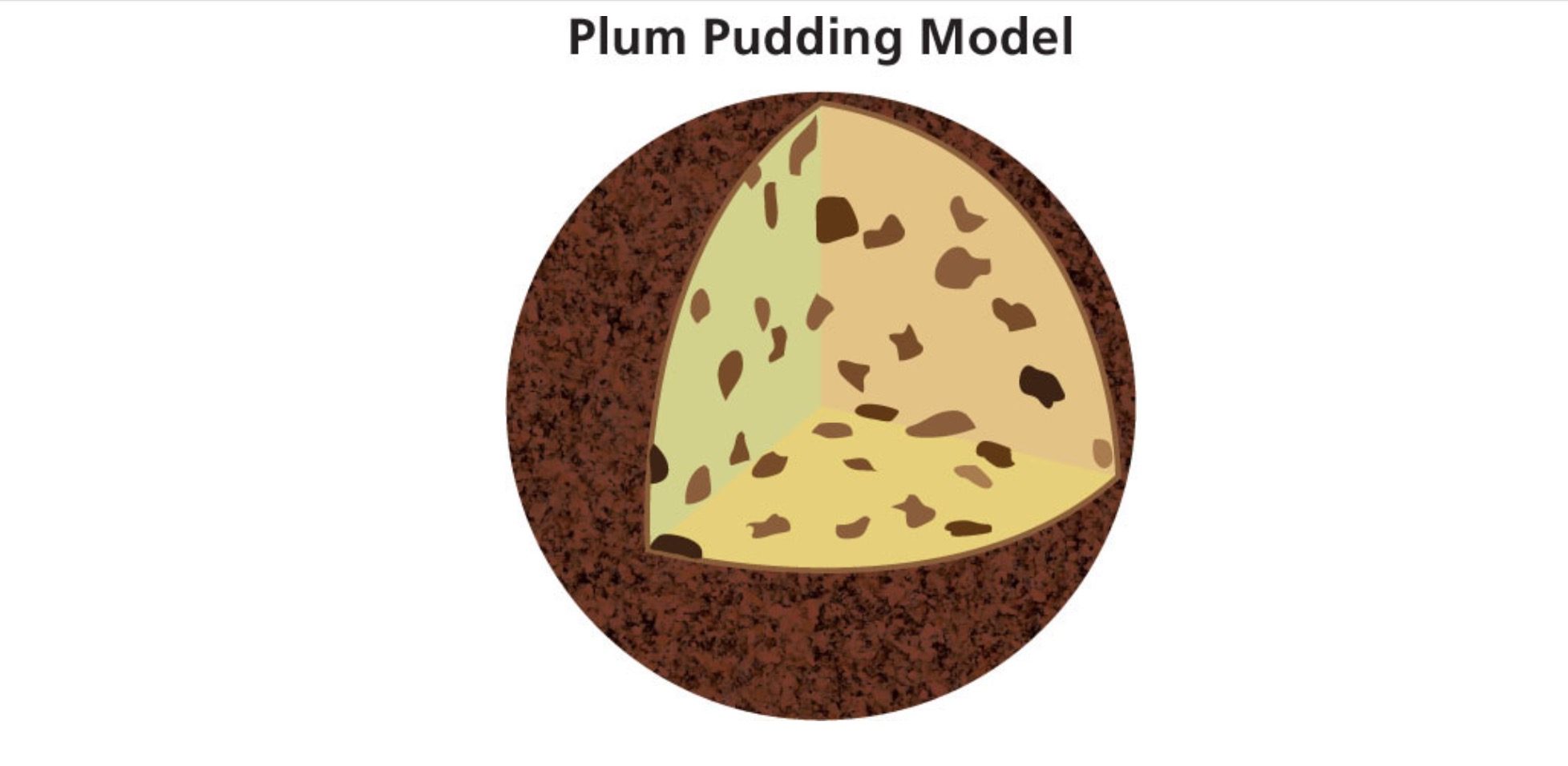
These Scientists Fired alpha particles at a piece of Gold foil. Some of the particles passed straight through but occasionally they were bounced back, indicating that they had encountered a sizeable obstruction with a large positive charge. The plum pudding model of the atom had dictated for years that the central core of an atom was a dispersed positive charge with electrons embedded in it, not the concentrated positive field experienced by the alpha particles in Rutherford's experiment. This led to the conclusion that the nucleus an atom existed of a central positive charge with electrons going around it.









 Thank you for your help!
Thank you for your help!